Torana™
The Torana™ Medical Imaging Gateway is a Class 1 software-based Medical Device (TGA / FDA) developed by SNAC for managing, routing and databasing medical scans in clinical and research settings. It is designed for secure, regulatory-compliant, automatic, and configurable transportation of clinical scans. Torana™ enables the seamless integration of complex data logistics, such as automatic image analysis, data archiving, anonymisation/re-identification into your daily clinical or research workflows. Additional features include sequence identification, protocol quality checking, and comprehensive audit trails under a CFR-21 Part 11 compliant framework.
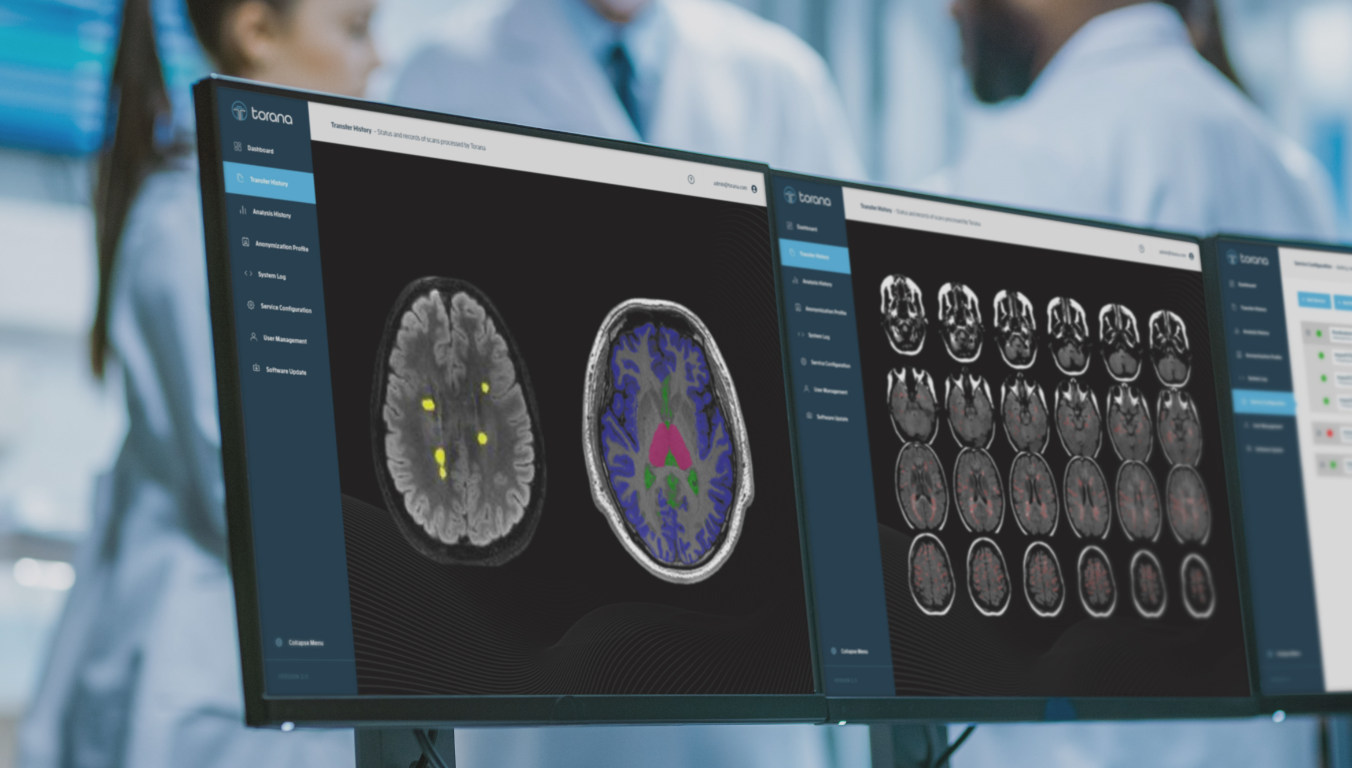
Torana™ comes with a user friendly web portal that allows non-technical staff to monitor scan transfer history and configure scan routing parameters and anonymisation profiles with a high degree of flexibility.
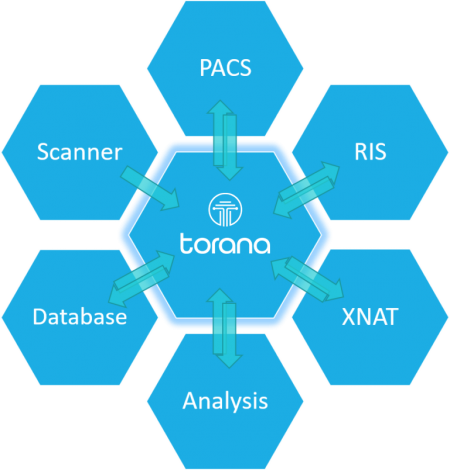
Torana™ communicates with various imaging and data entities.
Torana™ can listen to incoming images pushed from a scanner console, push or retrieve images from PACS systems through the DICOM protocol, transfer files and information through HTTP/HTTPS, and communicate with a RIS through HL7. Additionally, Torana™ can communicate with analysis servers, XNAT, and modern database systems for conducting complex image processing pipelines and research projects.
Automated image analysis is the next frontier in clinical radiology, yet penetration of AI- and traditional image analytics into daily clinical workflows is limited by poor integration and additional burden on clinicians. Torana™ connects in the background with a variety of data entities to silently trigger and route automated imaging analyses, supercharging productivity and accuracy in clinical radiology workflows. Newly acquired scans can be pushed (or auto-routed) to a local on-premises Torana™ instance. Torana™ can then de-identify and send the scans to a remote server for analyses such as the detection of anomalies or clinical metric computation. The analysis results are re-identified (on-site) and auto-routed to the local hospital/radiology clinic PACS and/or used to alert clinicians through the RIS.
Regulatory compliance
The key element of SNAC imaging informatics system Torana™ is listed as a FDA and TGA Class-I medical device which makes it reliable and safe for clinical use. The development of our informatics system is also certified to be ISO-13485 compliant.

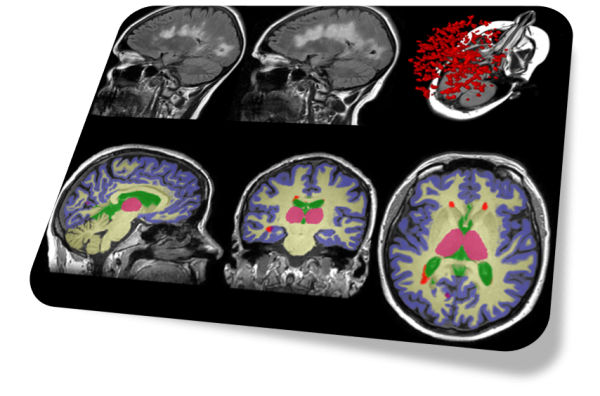
Image Analysis
SNAC also provides image analysis services for clinical or research users. Using Torana™ as the platform, clinicians or researchers can initiate image analysis requests from their PACS or local machines simply by sending images to Torana™ via a DICOM push. The images will be automatically de-identified, processed on a remote server, re-collected by Torana™ and re-identified, then routed back to the sender when the analysis has completed. Our analysis services encompass a variety of applications such as brain tissue segmentation, disease-specific reports, anomaly detection, etc. Torana™ also supports the integration with XNAT and modern database systems to allow researchers to easily archive and manage their analyses with comprehensive audit trails.
Supporting your customized workflow
Torana™ is highly flexible can be configured to support a wide variety of workflows. SNAC also provides comprehensive technical support to make sure you can customize Torana™ to suit your exact use cases. If you would like to use Torana™ in your department for clinical or research purposes, please contact us using the form below.