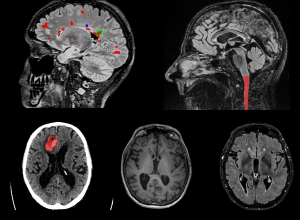
Precision neuro-imaging will deliver personalised diagnostic and treatment approaches for patients with chronic neurological disease.
Artificial intelligence (AI) is expected to transform both research and clinical neuro-imaging through automation and increased productivity, enhanced reporting accuracy and the rapid identification of critical abnormalities.
At SNAC, we are committed to developing Al algorithms and software platforms that seamlessly integrate with clinical radiology workflows – providing value add for clinicians without a single additional keystroke or mouse-click. SNAC has equipped with extensive experience in deep learning technology, including quantitative analysis (including patient triage and pathological segmentation), imaging generation (including image standardisation and synthesis), and large-scale AI model training and deployment, in the domain of neuro-imaging.
Quantitative AI – Segmentation
The extent of focal brain pathology provides clinicians with crucial insights into a patient’s neurological disease. For instance, the number and volume of lesions in the brain of patients with multiple sclerosis (MS) are key determinants of disease progression and response to therapy. However, manual segmentation of lesions by trained radiologists is a tedious and time-consuming process, typically resulting in only qualitative assessments in clinical practice.
Our advanced deep learning model, using AI-based segmentation techniques, can drastically reduce the time required to determine lesion volume—down to just 3 seconds. This innovative solution offers precise quantitative measurements, representing a significant saving in both time and cost, while making it possible to integrate lesion volume analytics into routine clinical workflows.
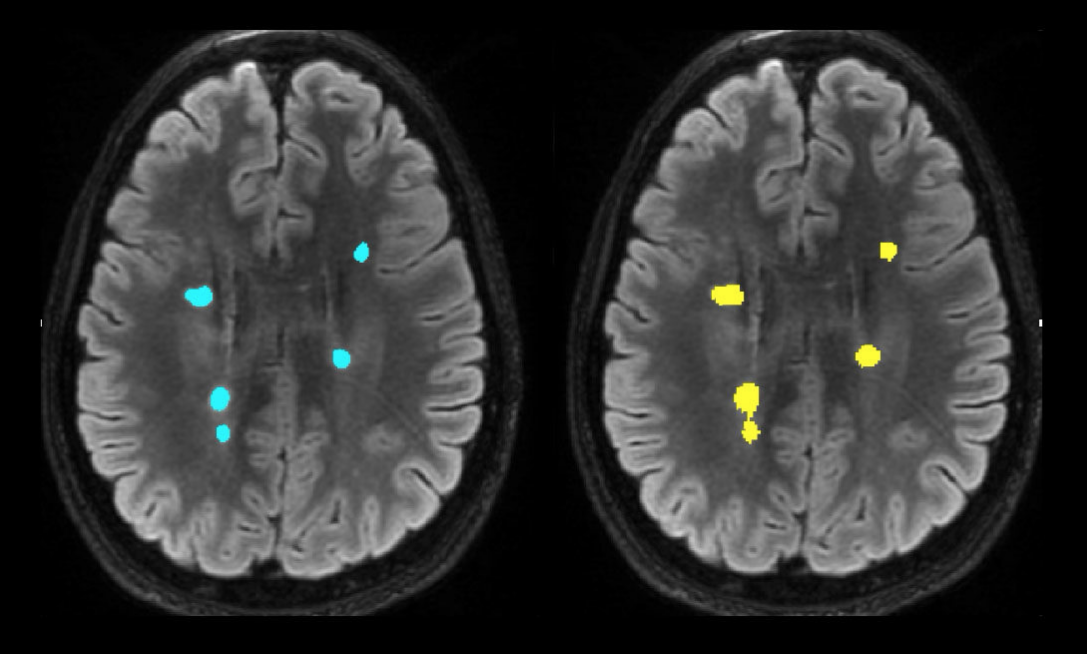
The image below provides a side-by-side comparison: manual MS lesion segmentation on the left, and SNAC’s fully automated AI-driven lesion segmentation on the right. Our breakthrough technology not only accelerates the segmentation process but also enhances patient care by enabling quantitative, data-driven treatment decisions.
Quantitative AI – Classification
Intracranial hemorrhage (ICH) is a medical emergency that demands immediate attention, but in busy hospital environments, delays in diagnosis can occur as radiologists review scans sequentially. These delays can be critical for patients needing urgent care.
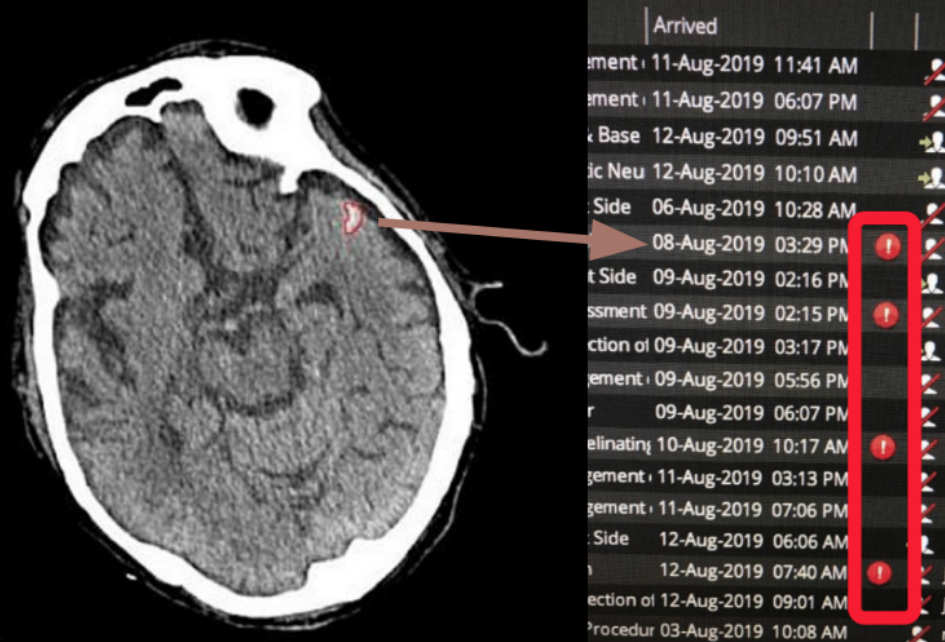
Deep learning AI algorithms developed by SNAC, trained with a large dataset of ‘golden labelled’ scans annotated by certified radiologists, robustly detect acute intracranial hemorrhage in CT images within seconds. Our advanced AI technology quickly identifies hemorrhages and automatically triages these cases, integrating results directly into the hospital’s Radiology Information System (RIS). This streamlined process ensures that life-threatening cases are prioritised, speeding up diagnosis and intervention.
By automating the detection and triage of critical conditions, SNAC’s AI solution significantly enhances workflow efficiency, enabling clinicians to respond faster and improving outcomes in time-sensitive medical situations.
Generative AI
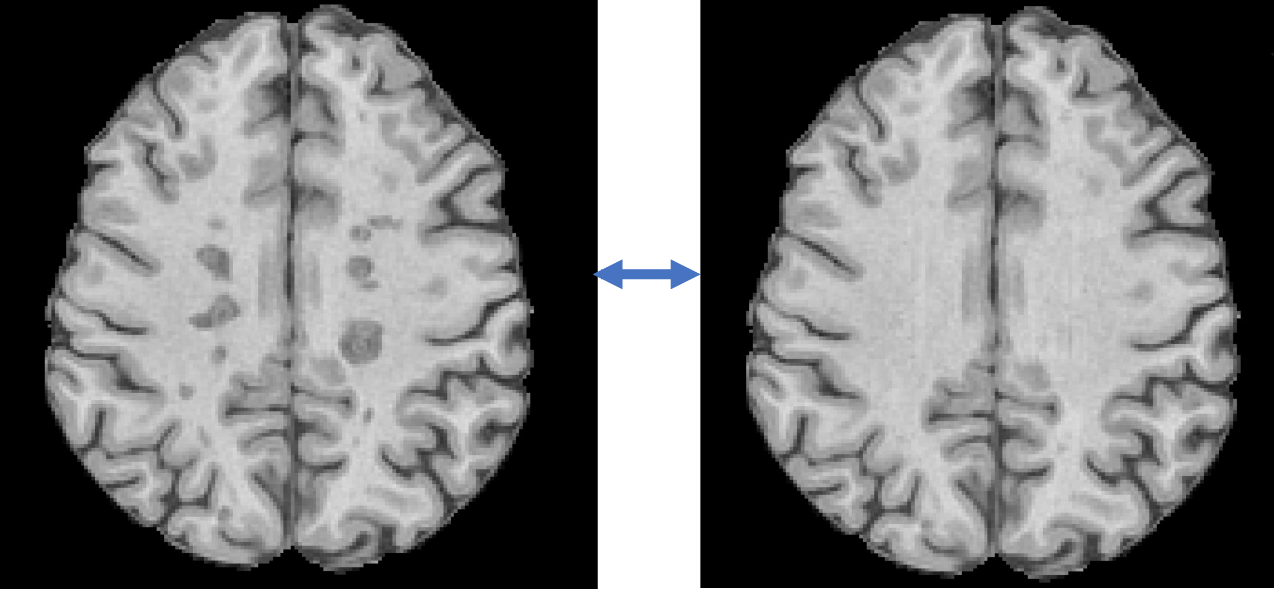
Volumetric analysis of brain tissues can be complicated by the presence of pathological conditions and the limitations of imaging protocols. For example, in multiple sclerosis, white matter lesions often appear as hypointense areas on T1-weighted MRI images, reflecting varying degrees of tissue injury. These lesions are frequently misclassified as grey matter or cerebrospinal fluid by automated segmentation algorithms, leading to biased brain substructure volume assessments.
SNAC’s advanced AI-based ‘inpainting’ techniques address this issue by augmenting and standardising images prior to analysis. By correcting these anomalies, our AI technology significantly improves the accuracy and consistency of brain volume measurements, ensuring more reliable results from automatic analysis pipelines.
Scalable AI
Volumetric analysis of brain tissues can be complicated by the presence of pathological conditions and the limitations of imaging protocols. For example, in multiple sclerosis, white matter lesions often appear as hypointense areas on T1-weighted MRI images, reflecting varying degrees of tissue injury. These lesions are frequently misclassified as grey matter or cerebrospinal fluid by automated segmentation algorithms, leading to biased brain substructure volume assessments.
SNAC’s advanced AI-based ‘inpainting’ techniques address this issue by augmenting and standardising images prior to analysis. By correcting these anomalies, our AI technology significantly improves the accuracy and consistency of brain volume measurements, ensuring more reliable results from automatic analysis pipelines.